Tunisia has positioned itself to profit from the increasing demand for cosmetic surgery — and not only from Tunisians. Many women from other countries, particularly in Europe, come to Tunisia in search of lower-cost operations. This trend has made Tunisia the “ second most popular African country for medical tourism,” reports Nadia Fenina, head of the Ministry of Health’s unit for health services exports.
Medical tourism agencies

Most patients coming from abroad go through tour companies to receive treatment. These groups offer packages that include meetings with health care providers and one or two days of operations, as well as hotel accommodation.
This is how Sophie* came to receive treatment in Tunisia. Sophie is a mother of three. After giving birth to her latest child, she begins to consider a breast lift and tummy tuck. In 2016, she looks online and discovers several options offered by Tunisian tourism agencies. She has her doubts, but she contacts them to learn more.
One agency, Estetika Tour, addresses her concerns by directing her to Facebook groups in which previous clients connect. In the groups, Sophie finds women who underwent similar procedures through the agency . . . and nothing but positive feedback. “ They all had good results, so I reserved a date and said ‘that’s it, I’m going,’” she recalls.
Audrey* and Céline* also came to Tunisia for breast surgeries. Like Sophie, they trusted Tunisian tour agencies to plan the logistics. All three women experienced complications following their operations. They claim to have received inadequate information from the tour agencies, and from their surgeons as well.
For all three women, the lead-up to the surgeries was relatively simple. To finalize a reservation, you complete an online questionnaire about your medical history and personal habits: previous operations, pregnancies, consumption of alcohol, smoking, etc. You are also asked to send a photo of your chest, so that a surgeon can make a remote pre-diagnosis.
Upon receiving this information, the agents send a quote, a proposed itinerary, and information on the type of operation and implant recommended by their surgeon. The patient is asked to get a blood test and a mammogram before arriving to Tunis so that the surgeon can operate right away.
The most commonly used breast implants fall into two categories: smooth and textured. For many years, health experts have warned against textured implants, which have been linked to many health issues, including the development of anaplastic large-cell lymphoma (ALCL), a rare type of cancer.
In response to these findings, most health organizations now advocate for the discontinued use of textured implants. However, many countries, including Tunisia, continue to use them anyway.
Sophie, Audrey, and Céline all received textured breast implants produced by Sebbin, a French implant manufacturer. They were not told that different types of breast implants were available, nor were they informed of the risks associated with textured implants in particular.
Minimizing risks

Tunisian companies that facilitate breast implant procedures offer little to no information about the risks involved. One such company, Estetika Tour, provides a fact sheet written by the French Society for Plastic and Reconstructive Surgery (SOFCPRE).
The fact sheet, written in 2015, vaguely mentions possible complications from breast augmentation, but the SOFCPRE says they’re rare and fails to provide a single statistic. It also fails to specify that textured implants are more likely to cause cancer than smooth implants.
With Estetika Tour, patients can forego the “ reflection period” that most healthcare providers recommend. Under French public health law, service providers are required to impose a 15-day delay between the delivery of a quote and the respective operation. There is no such requirement under Tunisian law.
Sophie, Audrey, and Céline all agreed that their surgeries in Tunisia felt expedited: they arrived, had a consultation with a surgeon — “ barely 10 minutes,” Sophie remembers —, and the next day, found themselves on the operating table.
Dr. Mounir Youssef Makni, president of the Tunisian National Council of Physicians, attributes the expedited timeline of these procedures to the medical tourism agencies who aim to reduce “ direct contact” between patients and healthcare providers. Many of his colleagues, he admits, do not find it “ acceptable that there is an intermediary between the doctor and the patient.” With so little time, the physiological state of the patient cannot be adequately assessed. “ It’s becoming almost industrial, and we are sacrificing quality,” he says.
“ Medicine should not be practiced in this manner. There must be dialogue between the patient and the doctor.”
To learn more about the risks of breast implants, we got on the phone with two medical tourism agencies: Estetika Tour and Medespoir. In both cases, company representatives defended the quality of the implants used. “ All of our prostheses are certified; they are internationally known brands and do not pose any risk,” claims Nadia,* on behalf of Medespoir.
An agent from Estetika Tour also confirms that their implants are made by Sebbin, the French manufacturer, and “ meet European standards.” When asked about associated risks of cancer, the agent admitted that there is an “ ongoing debate on the topic” and that “ the risk of cancer from textured implants is one in 3,000.” The responses from Estetika Tour perfectly echo information found in the SOFCPRE’s public fact sheet.
The Estetika Tour representative also claims that, considering “ the current state of the data, there is no need to suspend the use of textured implants in France.”
The National Agency for the Safety of Medicines and Health Products (ANSM) is expected to make a decision on the issue in February 2019, but for now, only textured implants manufactured by Allergan have been withdrawn from the European market. The decision was made on December 18th after it was revealed that an alarming rate of women with Allergan implants had developed cancer.
According to online lists published by the Ministry of Health’s Directorate of Pharmacy and Medicine (DPM), textured implants manufactured by Allergan were pulled from the Tunisian market several years ago. But other brands have remained. Textured implants from Arion, Eurosilicone, Sebbin, and Polytech are still available, and the risks associated with them are still unknown.
At the Doctor’s

Situated on the north-eastern edge of the capital, the Berges du Lac II neighborhood sees more and more commercial offices popping up. In this chic suburb of Tunis, the patient and surgeon meet for the first time. The consultation begins with general questions regarding the patient’s health and medical history. After a quick auscultation, the surgeon discusses the implant (size, shape, placement, etc.), outlines the operation, and provides post-operative instructions: “ no tobacco or alcohol — before or after — and no sports or physical activity.”
When asked about the possible risks, the doctor says that “ there is no operation without risk” and lists the possibilities of “ haemorrhage, infection, or a shell forming around the implant.” He says that implants are linked to cancer in “ about 50 cases out of millions of devices implanted.” This statistic corresponds to the number of ALCL cases detected in France since 2011. Worldwide, however, 656 cases of ALCL have been identified.
The doctor explains that he sources implants from “ Sebbin and Polytech, which are known brands,” and he proudly adds that his “ implants are micro-textured and thus are basically smooth implants.” The risks of cancer, he explains, are lower with his implants than with “ macro-textured implants.”
ICIJ researchers found that the distinction between “micro” and “macro” implants has no scientific backing. They also found that no studies have proven that “micro-textured” implants carry fewer risks.
According to a study conducted by the ANSM, the differences between “macro-textured” and “micro-textured” implants mainly serve marketing purposes; the classifications were made by the manufacturers themselves.
Inadequate follow-up
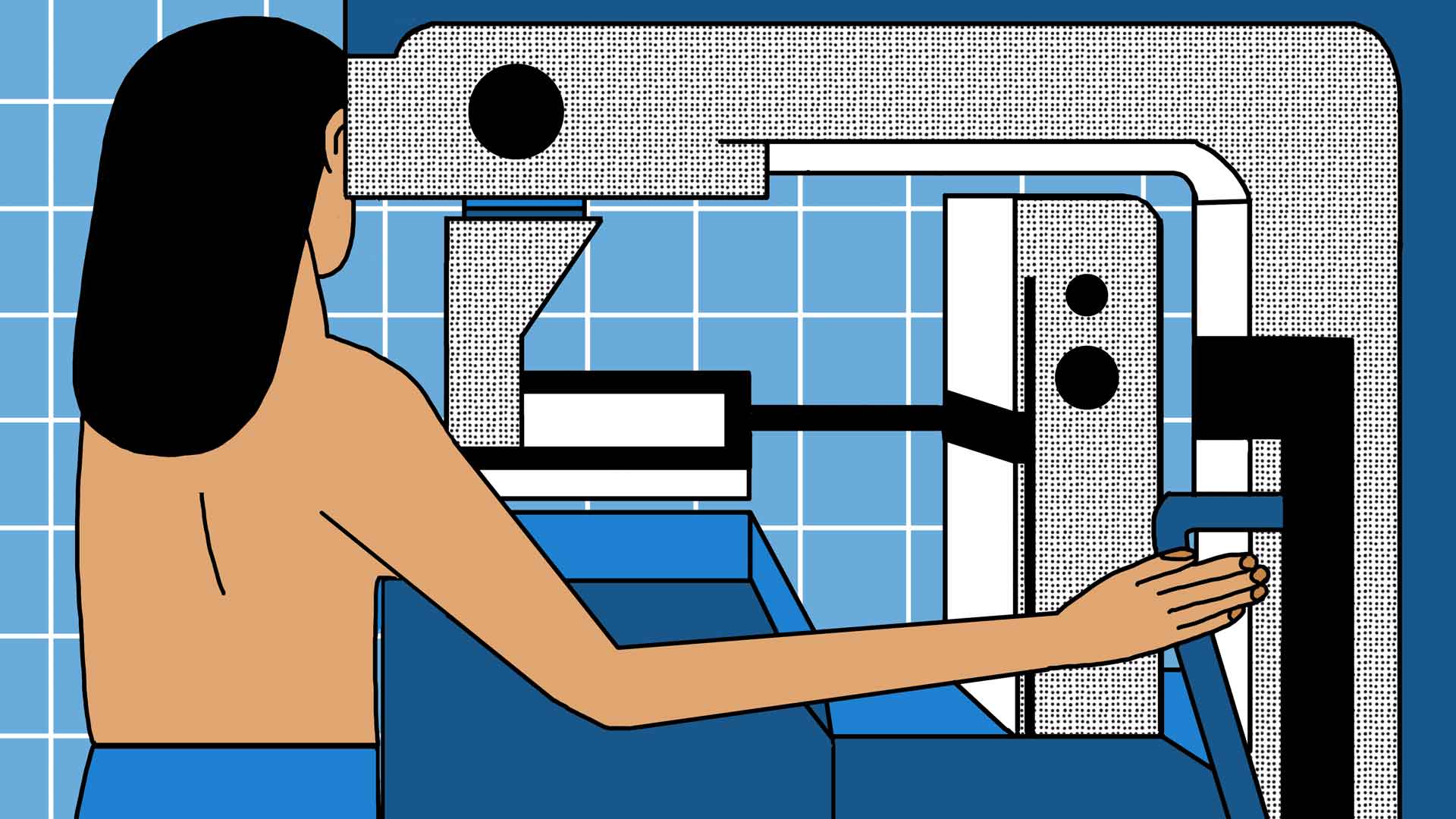
According to Tunisian tourism agencies and their surgeons, breast augmentation procedures require little post-operation rest or follow-up. Medespoir recommends “ 15 days of rest following the operation.” If the patient resides overseas, the company offers one month of communication with a surgeon over WhatsApp. The surgeon we consulted in Les Berges du Lac II claims that “ a breast ultrasound every year” is adequate follow-up.
Sophie begins to suffer four months after her surgery. Extreme pain in her right breast sends her to a doctor in France who administers an ultrasound and a mammogram. The problem is easy to diagnosis: one of her implants has folded and caused “a rupture.” Her right breast is misshapen; she needs to consult with a specialist immediately.
Sophie reaches out to the surgeon who treated her in Tunisia and explains the situation. He tells her to get an MRI to confirm the diagnosis. If confirmed, he would request a replacement implant from Sebbin and they would schedule another operation.
The mother of three finds a center that specializes in cases like her own. “ The doctor asked me how long I had the implant,” she recalls. “ She told me that it was not possible, that she had only seen this type of case with 10-year-old implants!”
The MRI shows that Sophie’s implant is creasing: the silicon gel contained within is dispersing and exacerbating the problem. The center advises Sophie to see a surgeon as soon as possible.
Sophie’s contract with Estetika Tour includes an eight-month guarantee, which allows her to return to Tunisia should problems with her implant arise. Sophie communicates with her surgeon over WhatsApp to figure out what to do next.
The surgeon informs her that he can’t do anything until Sebbin responds to him; he does not have any of their implants left in stock. Another four months pass before Sophie is able to change her defective implant. The pain amplified, and by the end, she could barely move her right arm. Neither the surgeon nor the agency provided her with a solution.
Who is responsible?
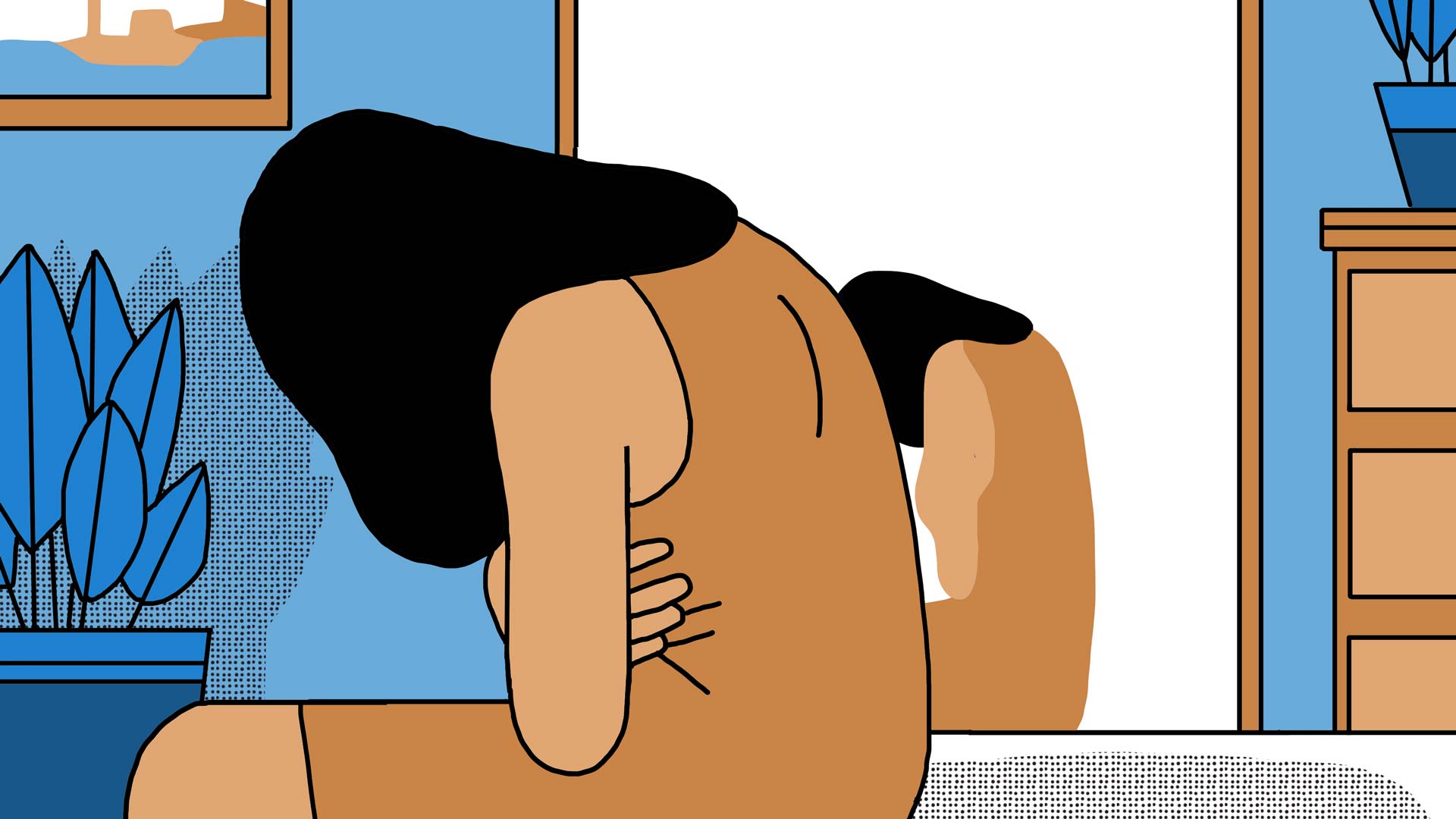
Sophie persists; she finally gets Estetika Tour to arrange another operation for her in Tunis. When she arrives to the clinic, the surgeon is surprised to see her. It seems as though the agency had not informed the surgeon of Sophie’s predicament. Nevertheless, they plan to operate the next day.
Sophie returns to France. Through WhatsApp, she stays in frequent contact with her surgeon. At one point, she accuses her surgeon of not having sufficiently warned her of the risks and complications associated with her surgery. The surgeon becomes aggressive and threatens to “ share pictures of her chest” if she continues to “ defame” him with her messages. We reached out to the surgeon in the hopes of finding out more about this potentially heinous betrayal of doctor-patient confidentiality. He failed to comment.
Once again, Sophie returns to Tunis for an operation that she hopes is her last. This time, she consults with various agencies and surgeons and does more research on their reputations. She still doesn’t know whether her complications are due to a defective implant or to her surgeon’s negligence. In an email addressed to Sophie’s doctor, a Sebbin representative claims that “ the implant’s manufacturing file meets official manufacturing standards.” Her surgeon suggests that some individual products in the batch could have been faulty, but the investigation stops there.
Audrey also suffered from creases developing in her textured implants from Sebbin. Her complications did not cause her extreme pain, but she decided to return to Tunisia to change her implants. A breast implant can last up to 10 years; Audrey’s lasted only two.
Céline suffered from something else. This time, it was not an implant deformation, it was her body fighting back. Céline’s body responded negatively to the implant, forming a casing around the foreign object. In this situation, as long as the casing doesn’t harden, and the pain is not untolerable, the implant can stay. But Céline feels off, and with her implants only three years old, she begins to worry.
But Céline cannot afford to be operated on in France. She tells herself she will make her way back to Tunis should her situation worsen. Sophie and Audrey, on the other hand, tried to have their follow-up operations in France, “ but the surgeons refused,” says Sophie. “ They don’t want to take the risk because in case of complications, it’s the last [surgeon] who is held responsible.”
Despite this risk, some surgeons in France do agree to operate on patients who have undergone surgeries in other countries. One such surgeon is Dr. Michael Atlan, who receives “ about two to three [patients] a month.” “ Most [of the patients] went to Tunisia where there are many packages [offered]. Others went to Morocco, and a few to Algeria,” says the surgeon.
According to him, there are many risks associated with rushed medical procedures, especially when they are performed far from a patient’s place of residency. “ The most common problems are infections, unhealed wounds, and unacceptable cosmetic results,” he says. Even if most of the results of these operations “ aren’t that bad,” the lack of proper follow-up remains “ a big problem.”
The doctor highlights another, often overlooked risk: travel by plane, only a few days after an operation. “ The risk of thrombosis or a pulmonary embolism is real. This [flying] should be forbidden,” he warns.
A lack of information, expedited operations in Tunisia, and inadequate postoperative follow-up . . . these three womens’ paths to receiving breast implants had many dangerous — potentially fatal — turns.
Sophie hopes to go address the issues she faced after her operation in Tunisia. She has contacted a lawyer in Tunisia in the hopes of filing complaints against her surgeon and the tour agency that organized the whole affair. She was led to believe their services were less risky than in reality. And she is not the only one. “ Several claims have been filed against cosmetic surgeons,” says Dr. Mounir Youssef Makni.
But these legal proceedings take time, and until “gross misconduct” is proven, surgeons under investigation can continue to operate as normal. Without sufficient evidence, the worst the surgeon can expect to receive is “a warning” from the Council of Physicians.
According to statistics provided by the Ministry of Health in 2013, roughly 376,000 people come to Tunisia for medical tourism every year. “ But this is an estimate, it’s very difficult to have an exact figure,” admits Nadia Fenina, who believes “ we can easily double or triple this figure.”
The Ministry of Health is working on a project to create a database of “ certified” medical tourism agencies, which would enable greater regulation of these agencies and better ensure the safety of patients from around the world. The president of the Council of Physicians claims this reform is necessary . . . so that the “ patient is no longer considered just as a statistic."




